Introduction
Minimal residual disease (MRD) monitoring is fundamental for risk stratification and treatment in acute lymphoblastic leukemia (ALL). Sensitive molecular assay requires presentation samples to be screened for immunoglobulin and T-cell receptor (IG/TCR) gene rearrangements. Previously samples were screened by PCR, heteroduplex analysis and Sanger sequencing to identify clone defining IG/TCR gene rearrangements. Subsequently these sequences were used to develop qPCR assays for MRD follow-up monitoring. More recently, targeted IG/TCR high-throughput sequencing (HTS) has been used with similar panels to identify gene rearrangements. Both these methods have limitations, in that only rearrangements covered by the panels are detected, and the techniques are labour intensive and expensive. Whole genome sequencing (WGS) has potential to discover all IG/TCR rearrangements in a single assay giving greater potential to detect markers for follow up MRD monitoring. In England all patients with ALL are eligible for WGS at diagnosis via the National Health Service, Genomic Medicine Service (NHS GMS).
Methods
Sixty-two patients, presenting with ALL at either Great Ormond Street Hospital or Bristol Children's Hospital, had short-read WGS via the NHS GMS performed concurrently with standard of care (SOC) targeted HTS to identify IG/TCR rearrangements. A bespoke bioinformatic pipeline was developed to identify IG/TCR rearrangements in the WGS samples. SOC targeted HTS analysis was performed using the open source Vidjil bioinformatic software. A cut-off of 5% (clone proportion of total clonotypes) was used to define an acceptable disease-specific clone for monitoring.
Results
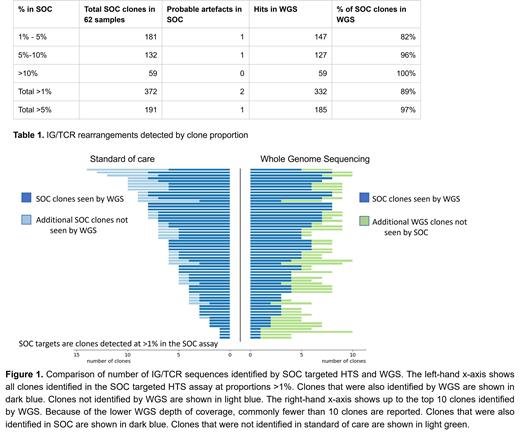
All 62 samples had a greater number of rearrangements detected by WGS than targeted HTS. The mean number of clones >5% detected was 2.7 by targeted HTS and 3.1 by WGS. For rearrangements identified by targeted HTS at a 5% cut-off, 97% (185 out of 190) were identified by WGS. All targets were identified by WGS using a 10% cut-off (Table 1).Despite the lower depth of coverage of the WGS assay, it retrieved 89% of sequences seen in SOC down to a level of 1%, as well as additional re-arrangements not seen by the SOC assay (Figure 1). As well as additional rearrangements being discovered by WGS, we observed novel rearrangements (dd3/ja rearrangements) not previously identified using a targeted approach. To establish if the additional sequences identified by WGS were clinically applicable as MRD targets, we designed allele specific oligonucleotides for a representative 32 rearrangements for testing by qPCR. Over half (n=16) were able to be used to track MRD in follow-up samples. New primer/probe sets were designed for the novel dd3/ja rearrangements identified by WGS, these were also tested by qPCR and performed well enough to be clinically applicable for MRD monitoring.
Conclusions
WGS approach outperformed targeted HTS, identifying more IG/TCR rearrangements suitable for molecular MRD monitoring in all patients. This is of particularly benefit in cases where targeted HTS only identifies 1 or 2 potential MRD targets. WGS also has the advantage of identifying other key gene rearrangements (BCR-ABL, KMT2A, SIL-TAL, IKZF1) that make excellent targets for MRD monitoring. Beyond this, WGS delivers all diagnostic information, germline and additional somatic findings that aid diagnosis. For ALL, in one test WGS can now replace all molecular diagnostics. In England all patients with leukemia are eligible for centralised WGS. With continued improvements in turnaround times, and reducing costs, we propose that WGS should be the new diagnostic standard for pediatric ALL
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal